About Clinical Studies - pdb
What are clinical studies?
Clinical studies (or clinical trials) are research studies designed to answer questions about investigational medications that may benefit patients. They study the safety and effectiveness of a product under rigorous U.S. Food and Drug Administration (FDA) guidelines. Through clinical studies, doctors and government regulators are able to learn about potential new medicines, medical devices and vaccines, and how to use them safely and effectively.
Sign up to match with a study

Make a difference: participate in a clinical study

If you have ever taken medication, you have benefited from clinical studies. Patients are a key part of clinical studies. Some volunteer for the chance to access potential new treatments for their medical conditions. Healthy volunteers may want to help advance medical science. Clinical research positively impacts the health of millions and can lead to new medications, vaccines and medical devices.
When you sign up for ICON Study Match at no cost, our clinical research specialists will review your information and inform you of studies you may be eligible for.
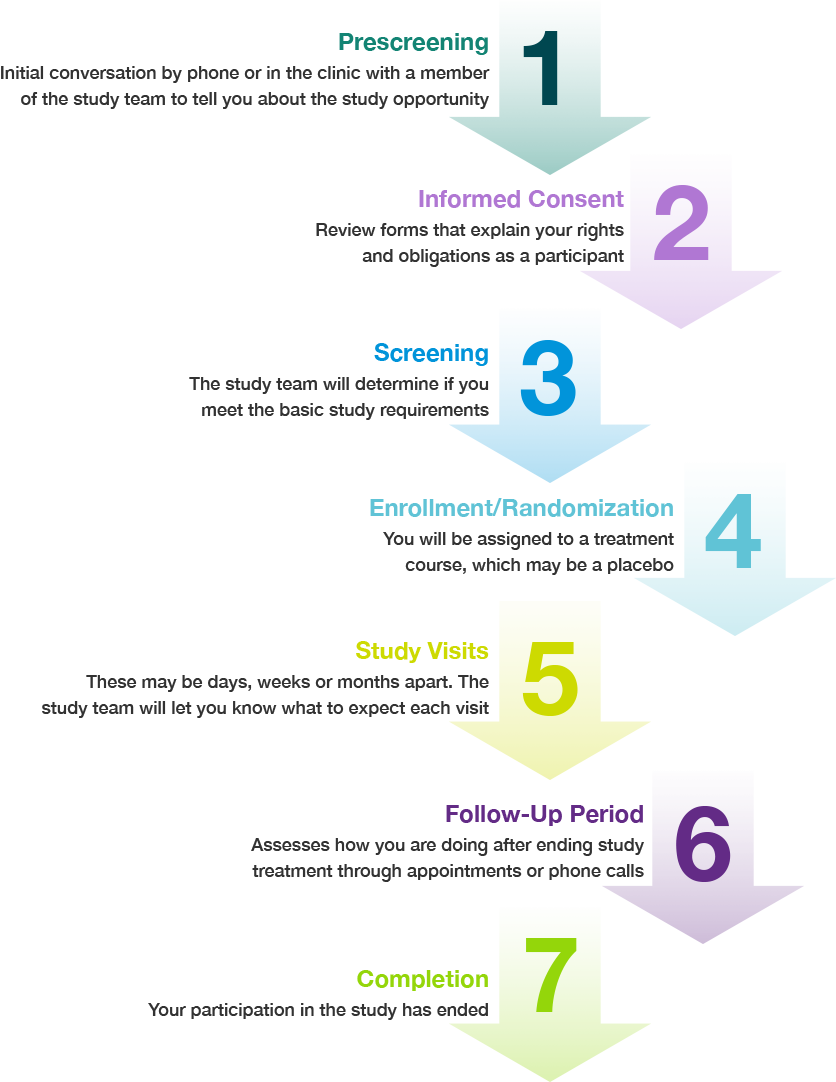
What is the clinical study process?
Benefits of participating in a clinical research study:
Chance to access otherwise unavailable investigational medications that may be an option for your condition
Receive close study-related care and monitoring by a study doctor at no cost
Help develop new treatments and make a positive impact on the health of patients
Receive reimbursement for your time and travel costs